 View Winners →
View Winners → 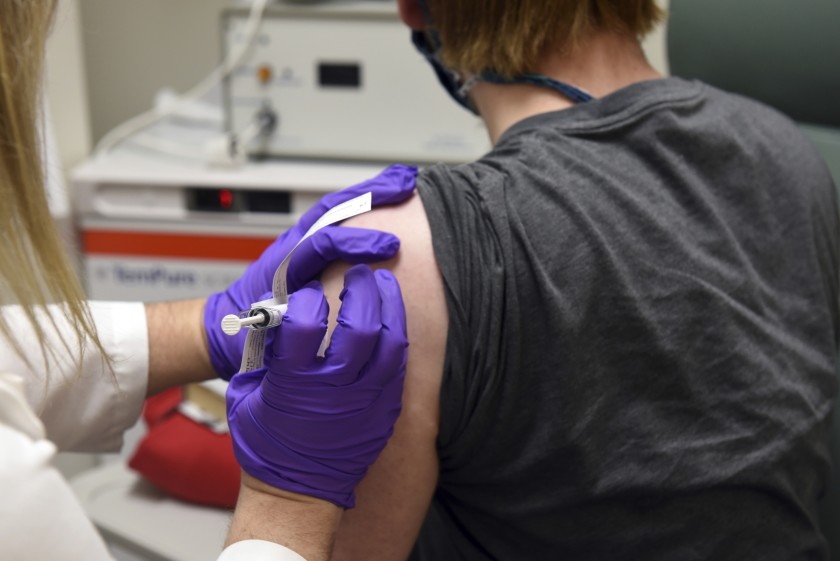
The first patient enrolled in Pfizer’s COVID-19 coronavirus vaccine clinical trial at the University of Maryland School. Pfizer says early data on its coronavirus vaccine candidate suggest the shots may be 90% effective at preventing COVID-19, indicating that the company is on track later this month to file an emergency-use application with U.S. regulators.
Monday’s announcement doesn’t mean a vaccine is imminent. This interim analysis, from an independent data monitoring board, looked at 94 infections recorded so far in a study that has enrolled nearly 44,000 people in the U.S. and five other countries.
Pfizer did not provide any more details about those cases, and cautioned that the initial protection rate might change by the time the study ends. Even revealing such early data is highly unusual. “We’re in a position potentially to be able to offer some hope,” Dr. Bill Gruber, Pfizer’s senior vice president of clinical development, told the Associated Press.
“We’re very encouraged.” Authorities have stressed that it’s unlikely any vaccine will arrive much before the end of the year, and limited initial supplies will be rationed. The shots made by Pfizer and its German partner, BioNTech, are among 10 possible vaccine candidates in late-stage testing […]
